The Clinical Trial Market Report 2025 explores the changing landscape of clinical trial technologies and their impact on drug development and healthcare innovation. Businesses are turning to trial solutions for study efficiency, regulatory compliance, and to speed up patient recruitment. This market outlook delves into key trends such as remote patient monitoring, immunotherapy, and clinical data management, redefining research across clinical trial domains. It also identifies investment opportunities and emerging startups that are shaping the future of clinical trials, paving the way for more patient-centric drug development processes.
This clinical trial outlook serves as a reference for stakeholders within the market, investors, policymakers, and economic analysts, providing a snapshot of the market’s health to map its trajectory for innovation and growth in the coming years.
StartUs Insights Clinical Trial Market Report 2025
- Executive Summary
- Introduction to the Clinical Trial Market Report 2025
- What data is used in this Clinical Trial Market Outlook?
- Snapshot of the Global Clinical Trial Market
- Funding Landscape in the Clinical Trial Market
- Who is Investing in the Clinical Trial Market?
- Emerging Trends in the Clinical Trial Market
- 5 Innovative Clinical Trial Startups
Executive Summary: Clinical Trial Market Report 2025
This report is created using data obtained from the Big Data and AI-powered StartUs Insights Discovery Platform, covering more than 4.7 million global companies, as well as 20K+ technologies and emerging trends. We also analyzed a sample of 1610+ clinical trial market startups developing innovative solutions to present five examples from emerging clinical trial market trends.
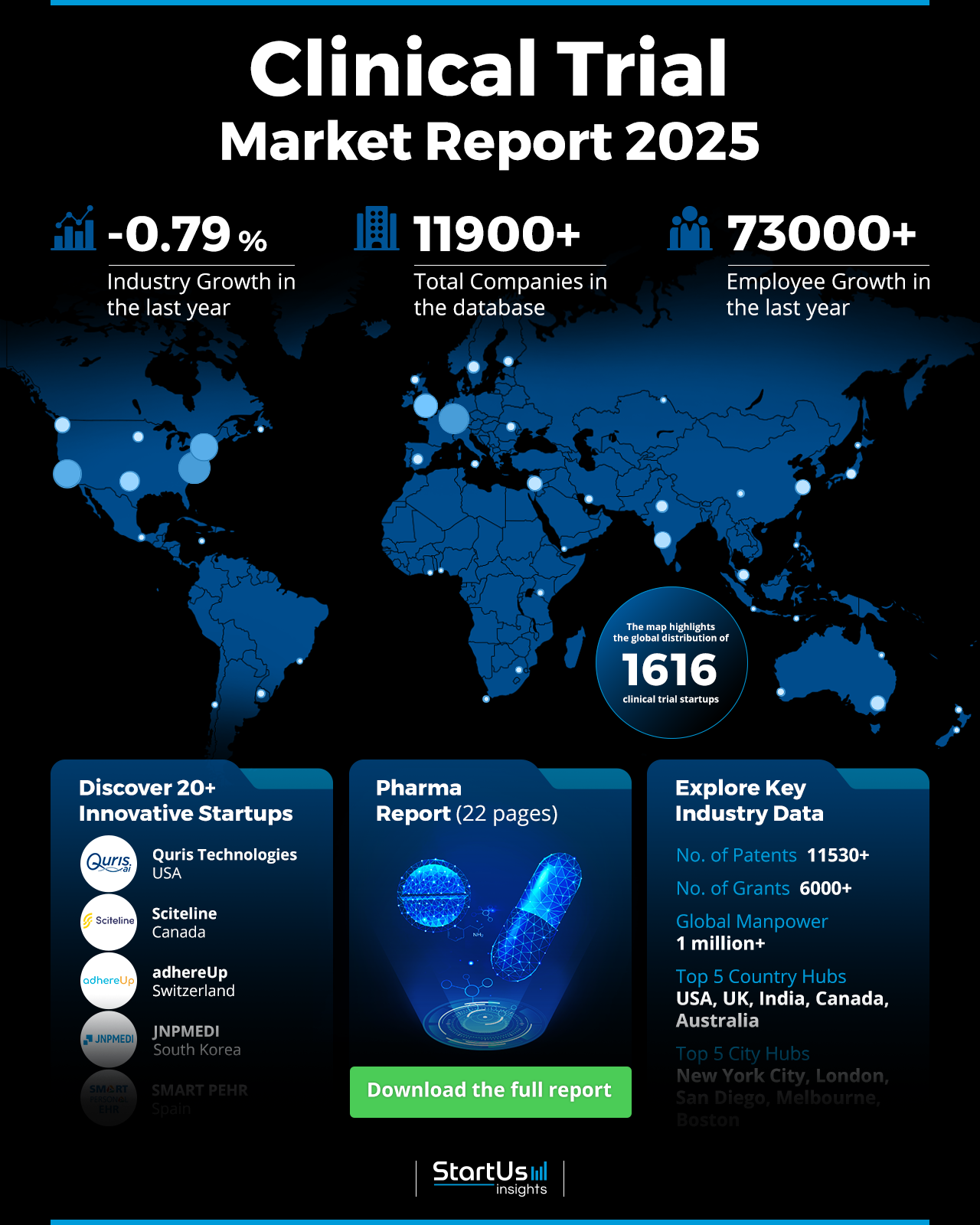
- Industry Growth Overview: The clinical trial market experienced an annual decline of 0.79%. More than 1610 startups are actively contributing to this domain.
- Manpower & Employment Growth: The market employs around 1 million professionals, with an increase of 73K+ employees in the past year.
- Patents & Grants: The clinical trial domain holds over 11530 patents and 6K grants. The yearly patent growth rate is 6.58%, with the USA and China leading the patent issuance.
- Global Footprint: Key country hubs include the USA, UK, India, Canada, and Australia, while major city hubs consist of New York City, London, San Diego, Melbourne, and Boston.
- Investment Landscape: The total funding rounds closed exceeds 18100, with average investment values exceeding USD 39 million per round. There are more than 9K investors actively involved.
- Top Investors: Key investors Eli Lilly, Roche, Boston Scientific, and more have actively invested over USD 27.9 billion.
- Startup Ecosystem: Five innovative startups, Quris Technologies (patients-on-a-chip), Sciteline (electronic data capture platform), adhereUp (remote pill monitoring), JNPMEDI (clinical trial data management), and SMART PEHR (personal electronic health record) showcase the market’s global reach and entrepreneurial spirit.
- Recommendations for Stakeholders: In the clinical trial market, stakeholders should improve data management by adopting artificial intelligence tools for real-time analysis. Implementing decentralized trial models for better accessibility will allow patients from diverse regions to participate more easily. In addition, collaboration with technology providers for secure and streamlined data integration will improve trial management. Governments should also create clear regulations that balance innovation, patient safety, and the use of emerging technologies in clinical trials.
Explore the Data-driven Clinical Trial Market Report for 2025
The clinical trial outlook 2025 uses data from the Discovery Platform and encapsulates the key metrics that underline the market’s dynamic growth and innovation. Our database contains 1610+ startups and over 11900 total companies which showcase a vibrant ecosystem in the clinical trial market. Despite a slight market decline of -0.79% over the past year, patent activity remains high with 11530+ patents and over 6K grants awarded. The clinical trial market’s workforce has grown by 73K+ employees, reaching a global total of 1 million employees. Further, the USA, UK, India, Canada, and Australia lead as the top five country hubs, while New York City, London, San Diego, Melbourne, and Boston dominate as leading city hubs for innovation and entrepreneurship.
What data is used to create this clinical trial market outlook?
Based on the data provided by our Discovery Platform, we observe that the clinical trial market ranks among the top 5% in the following categories relative to all 20K topics in our database. These categories provide a comprehensive overview of the market’s key metrics and inform the short-term future direction of the market.
- News Coverage & Publications: The clinical trial market received coverage in more than 24K publications in the last year.
- Funding Rounds: Our database includes information on over 18150 funding rounds.
- Manpower: The market employs more than 1 million workers, with over 73K new employees added in the past year.
- Patents: There are more than 11530 patents filed in this clinical trial domain.
- Grants: More than 6K grants have been awarded within the clinical trial market.
- Yearly Global Search Growth: The annual global search growth rate stands at 8.17% which reflects increasing demand for clinical trial solutions.
A Snapshot of the Global Clinical Trial Market
The clinical trial market saw a slight annual growth decline of 0.79%. Our database contains 1610+ startups, with over 970 in the early stages and 790 involved in mergers and acquisitions. In terms of patents, the clinical trial market holds 11530+ patents with 1010+ applicants. Plus, the yearly patent growth stands at 6.58%. The USA leads with 5590+ patents, followed by China with 1380+. This data underscores the ongoing innovation and evolving dynamics in the clinical trial market.
Explore the Funding Landscape of the Clinical Trial Market
The clinical trial market sees an average investment value of over USD 39 million per round. The market has attracted more than 9K investors, with over 18150 funding rounds closed. Additionally, these investments have supported more than 3470 companies which reflects continued investor interest and activity in the clinical trial domain.
Who is Investing in the Clinical Trial Market?
The combined investment value of the top investors in the clinical trial market exceeds USD 27.9 billion.
- Eli Lilly invested USD 4.3 billion across 22 companies.
- Roche invested USD 3.4 billion across 8 companies.
- Boston Scientific invested USD 3.1 billion across 5 companies.
- Johnson & Johnson invested USD 2.9 billion across 28 companies.
- Lundbeck invested USD 2.6 billion in at least 1 company.
- Sartorius Stedim Biotech invested USD 2.5 billion in at least 1 company.
- Asahi Kasei invested USD 2.4 billion across 6 companies.
- Ono Pharmaceutical invested USD 2.4 billion across 2 companies.
- Novo Nordisk invested USD 2.2 billion across 5 companies.
- Bayer invested USD 2.1 billion across 7 companies.
Access Top Clinical Trial Innovations & Trends with the Discovery Platform
Following are the emerging trends in the clinical trial market along with their firmographic details:
- Remote Patient Monitoring (RPM) is rapidly transforming clinical trials, through real-time patient data tracking and medication adherence. With over 1800 companies in this domain and 155K+ employees, this market is growing at an annual rate of 20.35%. The RPM market has seen a surge in workforce expansion, with 14K+ new employees joining in the last year.
- Immunotherapy continues to be a leading trend in clinical trials, particularly for cancer treatment. There are over 2250 companies operating in this market, employing 105K+ individuals, with 10K+ new employees joining in the past year. This domain is growing at an annual rate of 13.99%, driven by increasing research investments and developments in cancer immunotherapy.
- Clinical Data Management (CDM) plays a crucial role in providing accuracy, consistency, and regulatory compliance of clinical trial data. With 2240 companies and 126K+ employees, the market is growing steadily at 10.42% annually. Over 12K new employees joined CDM companies last year, reflecting the increasing need for skilled professionals to manage and analyze vast datasets generated during trials.
5 Top Examples from 1610+ Innovative Clinical Trial Startups
The five innovative startups showcased below are picked based on data including the trend they operate within and their relevance, founding year, funding status, and more. Book a demo to find promising startups, emerging trends, or industry data specific to your company’s needs and objectives.
Quris Technologies enhances Patients-on-a-Chip Technology
US-based startup Quris Technologies advances drug development with its Bio-AI Clinical Prediction Platform by integrating machine learning with patients-on-a-chip technology. This platform automates high-throughput testing of drug candidates on miniaturized organ systems. It generates predictive data that train machine learning algorithms to assess clinical safety and efficacy.
The startup incorporates genomic diversity by using stem cell-derived Patients-on-a-Chip for the platform to model human biological responses. Nano-sensors support the platform’s design by monitoring drug responses and collecting precise data. Further, Quris Technologies enables the selection of better drug candidates, reduces clinical trial failures, and delivers more effective treatments.
Sciteline offers an Electronic Data Capture Platform
Sciteline is a Canadian startup that develops an electronic data capture (EDC) platform to streamline clinical trial workflows. This platform employs a visual form builder that eliminates the need for programming and enables the rapid creation of electronic case report forms (eCRFs) within minutes. Its customizable interface offers study teams real-time visibility into study progress, participant status, and eCRF completion. The platform also has built-in tools for quick search, filtering, and sorting.
Dedicated workflows allow primary investigators and quality control specialists to review and sign off on submitted eCRFs efficiently. In addition, the digital audit history interface visually tracks and organizes data changes for compliance. Further, the platform’s self-serve administration panel supports user management, access control, and branding customization to align with organizational needs. Moreover, it includes automated triggers and alerts to flag discrepancies during data capture.
adhereUp renders Remote Pill Monitoring
adhereUp is a Swiss startup transforming clinical trials with its Blistee medication tracking system. It enables real-time monitoring of pill intake to optimize patient adherence to medical protocols. The system begins with pharmacist-approved blister packing of tablets, which are delivered directly to patients’ homes. Patients self-register through the Blistee mobile app and gain access to a digital platform and web portal for remote follow-up. The Blistee system tracks intake time and drug type while providing alerts and reminders for adherence. Further, through precise monitoring with accessible tools, adhereUp manages medication, streamlines clinical trials, and supports data collection to improve trial outcomes.
JNPMEDI avdances Clinical Trial Data Management
South Korean startup JNPMEDI develops Maven CDMS, a platform for clinical trial data collection and management. It integrates essential functions such as electronic data capture (EDC) and professional services such as investment and licensing services to streamline clinical trial operations. The platform offers various modules, including Maven Trigger for auto/manual query configuration, and Maven Coder for global medical coding.
Its Maven RTSM module is used for randomization and investigational product (IP) supply management. Additionally, Maven Auditor provides blockchain-based change history management for data integrity. The Maven eCOA module supports remote clinical trials to improve patient and clinician convenience. Also, Maven Docs simplifies document creation, viewing, and approval with electronic signatures. Further, the Maven Safety solution optimizes pharmacovigilance tasks, and Maven Builder aids in clinical trial database design.
SMART PEHR streamlines Personal Electronic Health Record
Spanish startup SMART PEHR develops a patient-centric platform that centralizes medical records into a single personal electronic health record (PEHR). This platform aggregates data from electronic medical records (EMR), electronic clinical outcome assessment (eCOA), telehealth, wearables, DNA sequencing, and more. By leveraging artificial intelligence (AI), machine learning (ML), and natural language processing (NLP), SMART PEHR analyzes this real-world data (RWD) to uncover patterns, detect anomalies, and predict health risks in real time.
This platform also enables interoperability across systems using HL7 FHIR standards for the accessibility and usability of healthcare data. Besides, SMART PEHR’s integration of RWD with clinical trial data allows life sciences and pharmaceutical companies to conduct more decentralized real-world trials.
Gain Comprehensive Insights into Clinical Trial Trends, Startups, or Technologies
In 2025, the clinical trial market will experience growth as developments in real-world data (RWD), artificial intelligence (AI), and machine learning (ML) are changing the way trials are conducted. These technologies will drive the shift towards decentralized and real-world trials while improving patient recruitment, and trial efficiency, and accelerating the drug development process. Get in touch to explore all 1610+ startups and scaleups, as well as all market trends impacting 11900+ companies.









